Test product spiked at low degrees with representative organisms (including the aerobic bacterium Staphylococcus aureus, spore forming bacterium Bacillus subtilis, anaerobic bacterium Clostridium sporogenes or even the fungus Candida albicans) are employed to be sure there is not any inhibitory effect through the sample which can mask contaminants, so as to validate the test.
This minireview offers an overview of this sophisticated discipline of recent great producing techniques (cGMP) dependant on biopharmaceutical industry expectations and summarizes the compendial and different rapid microbial test methods accessible for product or service sterility and Mycoplasma
Good assembly from the filling gear is essential to ensure the successful filling of items into vials, ampoules, or pre-loaded syringes (see Figure 3). The sterilization of the filling assembly ought to be validated, and transfers within the autoclave to your filling equipment should go through a cellular laminar airflow device to stop contamination.
The data collected including the amount site visitors, the source the place they have got come from, and also the pages frequented in an anonymous variety.
This web site isn't going to exist inside your selected language. Your desire was saved and you may be notified once a web site can be seen get more info inside your language.
Include classes acquired and most effective procedures to repeatedly evolve the RCFA system and optimize its impact on the organization.
Obtain this element partnership: Continual production: an evolving technological innovation for drug substance producing
5.2.thirteen In the event the test is declared being the invalid repeat with the similar number of the device as in the initial test. It no evidence of microbial progress is present in the repeat test, the planning remaining examined complies Using the test for sterility.
If you want to to comment on The existing information, make sure you make use of the 'Written content Feedback' button beneath for Recommendations on getting in contact with the issuing company
Sturdy sterility testing protocols are essential to secure patients, medicine provide chains and companies’ base traces. But how do companies Establish profitable sterility processes?
Direct inoculation or membrane filtration. Membrane filtration may possibly support get more info in eradicating prospective society inhibitors.
I would really like to join newsletters from Sartorius (Sartorius AG and its affiliated businesses) based mostly of my own interests.
You can alter your cookie and related details processing preferences Anytime by way of our "Cookie Options". You should see our Cookie Coverage To find out more about using cookies on our Web site.
We are trying our best to create This website user-welcoming and resourceful with well timed/up-to-date specifics of each pathogen, disease caused by them, pathogenesis, and laboratory analysis.
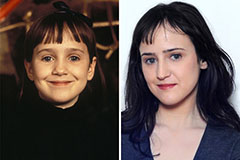 Mara Wilson Then & Now!
Mara Wilson Then & Now! Patrick Renna Then & Now!
Patrick Renna Then & Now! Mackenzie Rosman Then & Now!
Mackenzie Rosman Then & Now!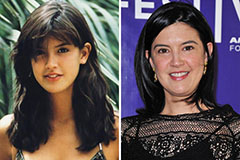 Phoebe Cates Then & Now!
Phoebe Cates Then & Now! Brooke Shields Then & Now!
Brooke Shields Then & Now!